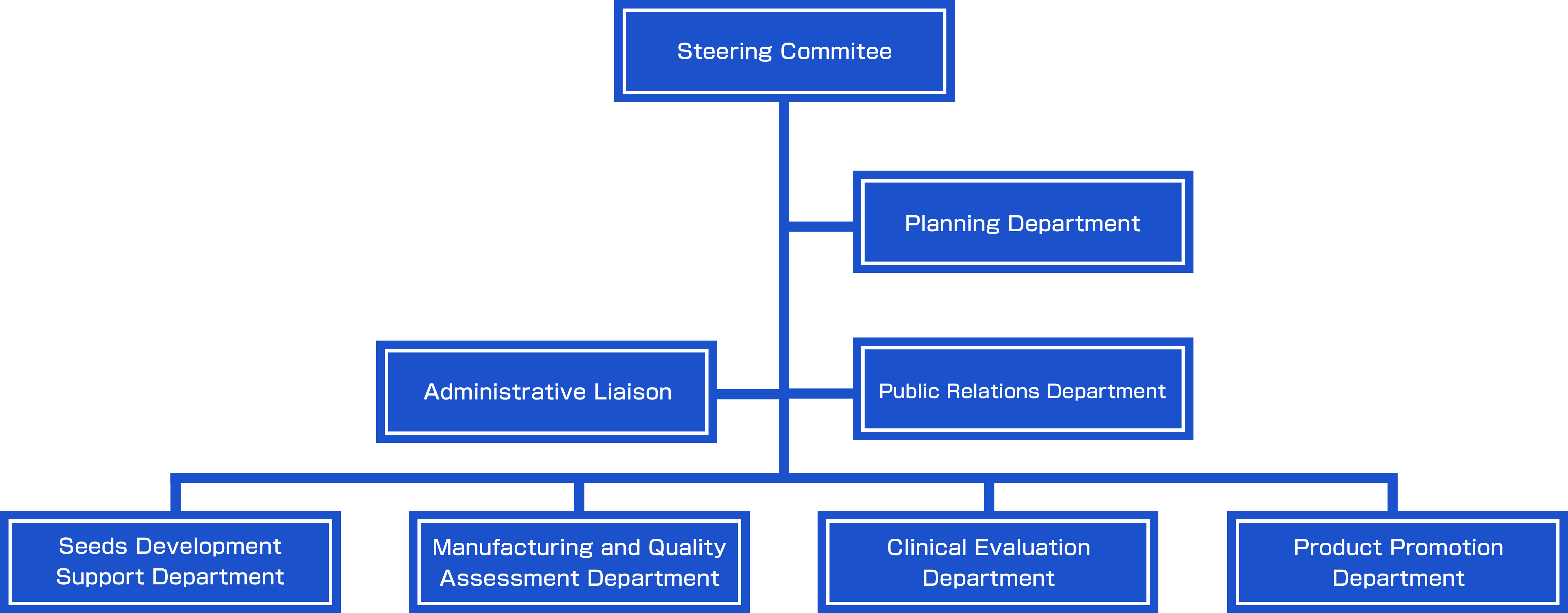
Organizational Chart
Provision System
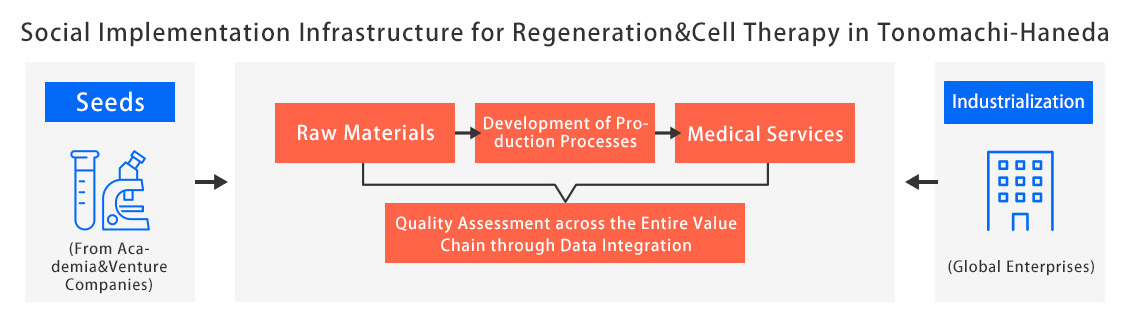 Building on cells provided by university hospitals, research institutes in Tonomachi and Haneda will analyze and standardize their characteristics. Once scientifically grounded criteria for cell quality evaluation are established, we will develop regenerative medical products and disseminate them through medical institutions. By incorporating patient outcomes from clinical sites, we aim to create an ecosystem that continually drives the development of even better regenerative medical products.
Building on cells provided by university hospitals, research institutes in Tonomachi and Haneda will analyze and standardize their characteristics. Once scientifically grounded criteria for cell quality evaluation are established, we will develop regenerative medical products and disseminate them through medical institutions. By incorporating patient outcomes from clinical sites, we aim to create an ecosystem that continually drives the development of even better regenerative medical products.
Affiliated Institutions
Collaborating Institutions
Inter-Site Collaboration
Management Office

Keio University Tonomachi Town Campus
Research Gate Building TONOMACHI 2-4A, 3-25-10 Tonomachi, Kawasaki-ku, Kawasaki, Kanagawa 210-0821 Japan
TEL:+81 (0)44-201-7466
FAX:+81 (0)44-201-7467

FUJITA MEDICAL INNOVATION CENTER TOKYO
1-1-4, Hanedakuko, Ota-ku, Haneda Innovation City Zone A 144-0041 Tokyo, Japan
TEL:+81-3-5708-7830