2025/07/18
Notice
Prof. Yoshimi Yashiro, Head of the Planning Division, published a paper in July as the first and corresponding author.
Prof. Yoshimi Yashiro, Head of the Planning Division, published a paper titled “Evaluation of the usage of more than 50 sheets of autologous cultured epidermis (JACE®) sheets in treating patients with severe burns beyond insurance coverage limits“, in July as the first and corresponding author.
Summary
Autologous cultured epidermis, JACE®, Japan’s first regenerative medical product, is used as the standard treatment for wound closure in patients with severe burns. Although this product is listed for national health insurance in Japan, medical institutions place orders for JACE® beyond the limit of 50 sheets calculated for insurance coverage (insurance coverage limit) for lifesaving purposes.
The authors planned and conducted a non-interventional survey on the usage of JACE® from June 21, 2023, to February 29, 2024. In cases where more than 50 sheets of JACE® were used for patients with severe burns, information obtainable at the manufacturing stage and post-treatment feedback from physicians in charge were collected, and considered the number of insurance required for JACE®.
Click here for more details.
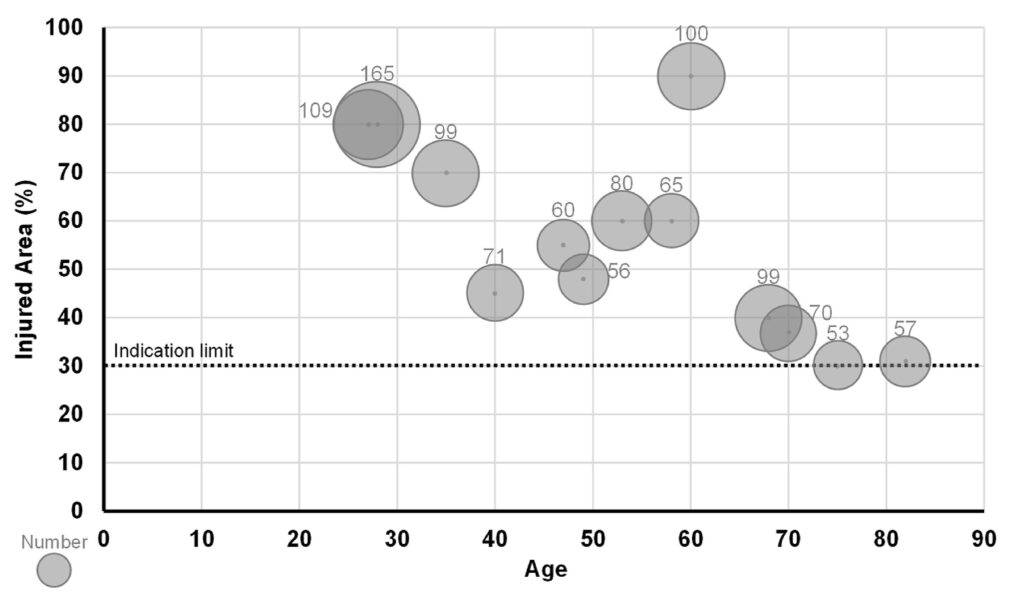
Fig. 1. Age, injured area, and number of sheets used in group H (patients who received 51 or more JACE® sheets).
(Reference:Yoshimi Yashiro, Masukazu Inoie, Evaluation of the usage of more than 50 sheets of autologous cultured epidermis (JACE®) sheets in treating patients with severe burns beyond insurance coverage limits, Regenerative Therapy, Volume 30, 2025, Pages 415-420, ISSN 2352-3204, https://doi.org/10.1016/j.reth.2025.07.005.)
